MTA-Cooperative PRMT5 Inhibitor
(MRTX1719)
Mirati is advancing new research to address MTAP-deleted cancers with the goal of providing a potentially novel treatment option both as a monotherapy and in combination with other agents.
(MRTX1719)
Mirati is advancing new research to address MTAP-deleted cancers with the goal of providing a potentially novel treatment option both as a monotherapy and in combination with other agents.
Scientific Rationale
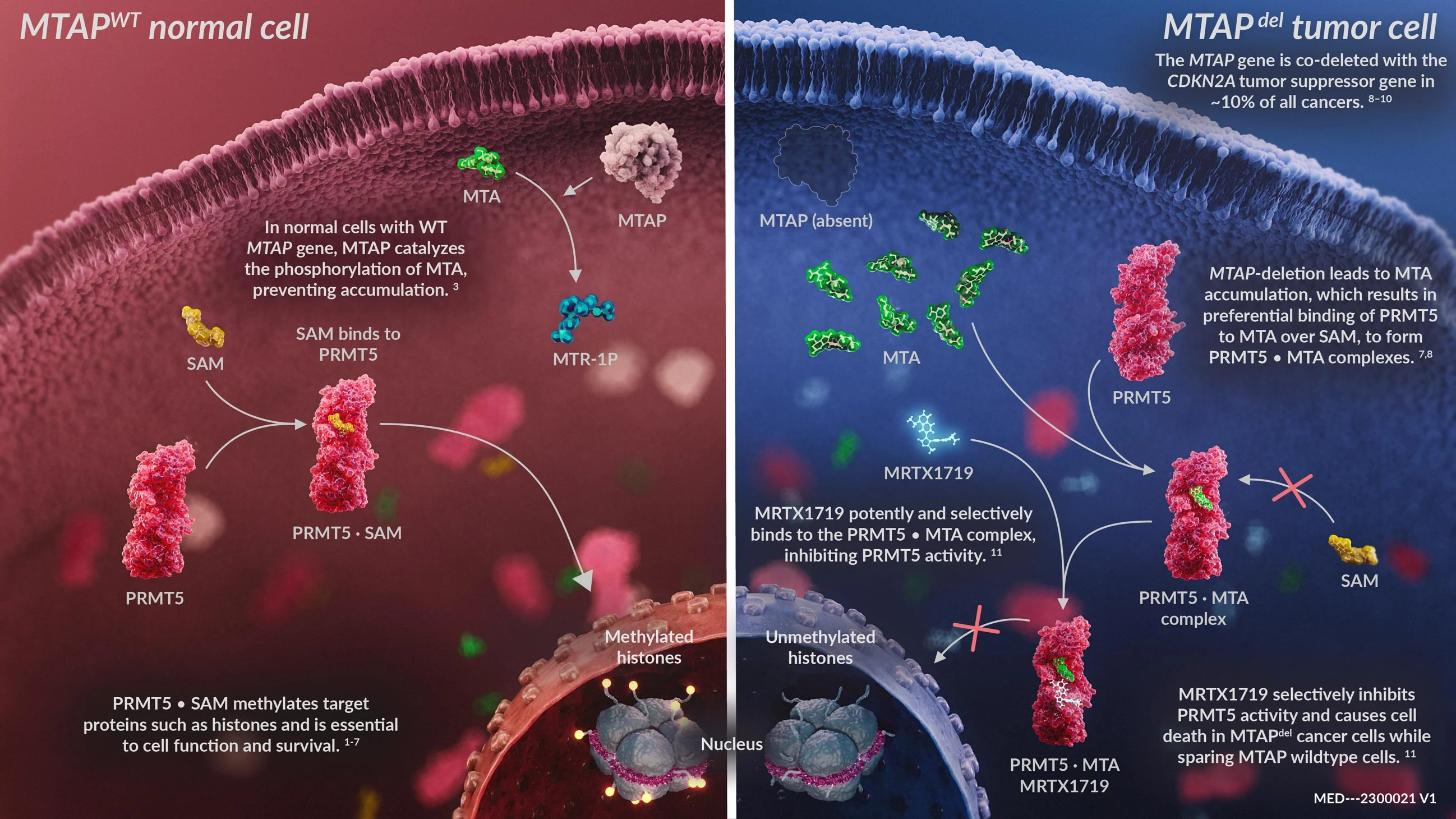
Methylthioadenosine Phosphorylase or MTAP is a gene that frequently suffers homozygous deletion in cancer which results in the accumulation of methylthioadenosine (MTA) within cancer cells. MTA binds to PRMT5 and forms the PRMT5-MTA complex. This new complex creates a novel drug target for the potential treatment of MTAP-deleted cancers.
Cancer cells with MTAP deletion were hypothesized to be sensitive to inhibition of the PRMT5-MTA complex.17,18,19 This is an example of synthetic lethality where the inhibition of two genes results in cell death.
Unmet Need
MTAP homozygous deletion occurs in around 10 percent of cancers,20 including a high percentage of non-small cell lung cancer (NSCLC), pancreatic cancer and mesothelioma. MTAP-deleted cancers are associated with a poor prognosis; median survival rates among pan-cancer patients with tumors harboring homozygous co-deletions of 9p21 are significantly lower than the tumors with wildtype 9p21 (24 months vs. 115 months),20 representing a significant unmet medical need.
By the numbers:
~10% of all cancers have CDKN2A/MTAP deletion
MRTX1719
Mirati is advancing new research to address MTAP-deleted cancers with the goal of providing a potentially novel treatment option both as a monotherapy and in combination with other agents.
MRTX1719 is an investigational, internally discovered MTA-cooperative PRMT5 inhibitor that selectively binds the PRMT5-MTA complex, inhibiting PRMT5 function in MTAP-deleted cancer cells. MRTX1719 selectively inhibits the viability of cancer cells with MTAP deletion and is designed to spare healthy, non-tumor cells.21 In pre-clinical studies, MRTX1719 induced tumor regression in cell line and patient-derived xenograft tumor models.22 This differentiated approach may demonstrate a potentially improved therapeutic index in preclinical studies relative to first generation PRMT5 inhibitors.
Mechanism of Action