Programs
Our fearless pursuit of science, guided by in-depth translational research, aims to address unmet needs for patients with limited treatment options.
Our fearless pursuit of science, guided by in-depth translational research, aims to address unmet needs for patients with limited treatment options.
MRTX1719
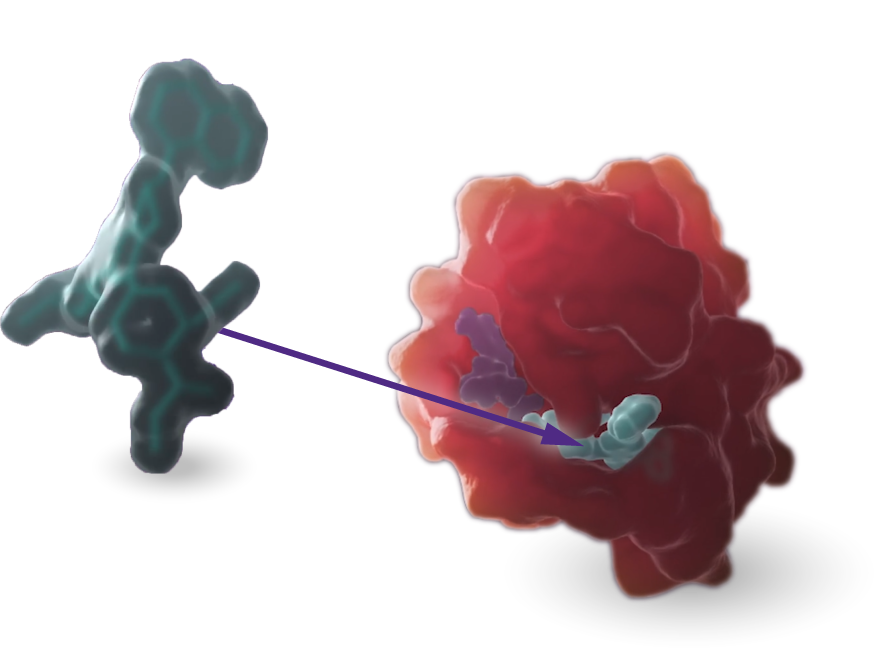
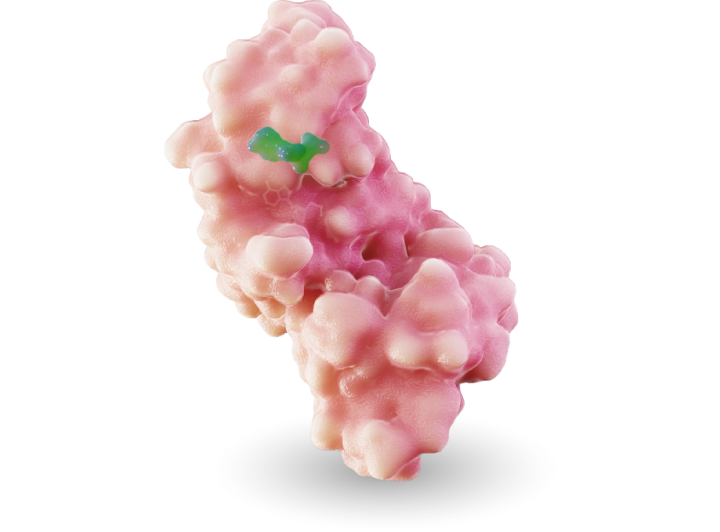
MRTX1719 is an investigational, internally discovered synthetic lethal PRMT5 inhibitor for the treatment of methylthioadenosine phosphoylase (MTAP)-deleted cancers.